Page 15 - Demo
P. 15
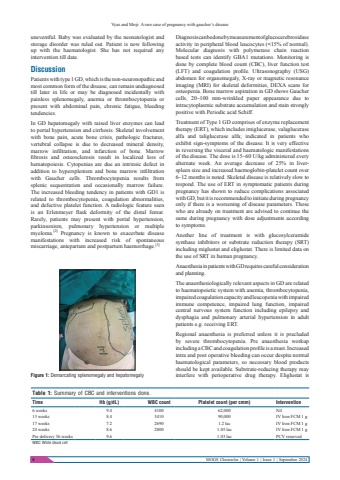
Vyas and Mirji: A rare case of pregnancy with gaucher%u2019s disease8 MOGS Chronicles | Volume 1 | Issue 1 | September 2024uneventful. Baby was evaluated by the neonatologist and storage disorder was ruled out. Patient is now following up with the haematologist. She has not required any intervention till date.DiscussionPatients with type 1 GD, which is the non-neuronopathic and most common form of the disease, can remain undiagnosed till later in life or may be diagnosed incidentally with painless splenomegaly, anemia or thrombocytopenia or present with abdominal pain, chronic fatigue, bleeding tendencies.In GD hepatomegaly with raised liver enzymes can lead to portal hypertension and cirrhosis. Skeletal involvement with bone pain, acute bone crisis, pathologic fractures, vertebral collapse is due to decreased mineral density, marrow infiltration, and infarction of bone. Marrow fibrosis and osteosclerosis result in localized loss of hematopoiesis. Cytopenias are due an intrinsic defect in addition to hypersplenism and bone marrow infiltration with Gaucher cells. Thrombocytopenia results from splenic sequestration and occasionally marrow failure. The increased bleeding tendency in patients with GD1 is related to thrombocytopenia, coagulation abnormalities, and defective platelet function. A radiologic feature seen is an Erlenmeyer flask deformity of the distal femur. Rarely, patients may present with portal hypertension, parkinsonism, pulmonary hypertension or multiple myeloma.[2] Pregnancy is known to exacerbate disease manifestations with increased risk of spontaneous miscarriage, antepartum and postpartum haemorrhage.[3]Diagnosis can be done by measurement of glucocerebrosidase activity in peripheral blood leucocytes (<15% of normal).Molecular diagnosis with polymerase chain reaction based tests can identify GBA1 mutations. Monitoring is done by complete blood count (CBC), liver function test (LFT) and coagulation profile. Ultrasonography (USG) abdomen for organomegaly, X-ray or magnetic resonance imaging (MRI) for skeletal deformities, DEXA scans for osteopenia. Bone marrow aspiration in GD shows Gaucher cells, 20%u2013100 mm-wrinkled paper appearance due to intracytoplasmic substrate accumulation and stain strongly positive with Periodic acid Schiff.Treatment of Type 1 GD comprises of enzyme replacement therapy (ERT), which includes imiglucerase, valaglucerase alfa and taliglucerase alfa; indicated in patients who exhibit sign-symptoms of the disease. It is very effective in reversing the visceral and haematologic manifestations of the disease. The dose is 15%u201360 U/kg administered every alternate week. An average decrease of 25% in liverspleen size and increased haemoglobin-platelet count over 6%u201312 months is noted. Skeletal disease is relatively slow to respond. The use of ERT in symptomatic patients during pregnancy has shown to reduce complications associated with GD, but it is recommended to initiate during pregnancy only if there is a worsening of disease parameters. Those who are already on treatment are advised to continue the same during pregnancy with dose adjustments according to symptoms.Another line of treatment is with glucosylceramide synthase inhibitors or substrate reduction therapy (SRT) including miglustat and eliglustat. There is limited data on the use of SRT in human pregnancy.Anaesthesia in patients with GD requires careful consideration and planning.The anaesthesiologically relevant aspects in GD are related to haematopoietic system with anemia, thrombocytopenia, impaired coagulation capacity and leucopenia with impaired immune competence, impaired lung function, impaired central nervous system function including epilepsy and dysphagia and pulmonary arterial hypertension in adult patients e.g. receiving ERT.Regional anaesthesia is preferred unless it is precluded by severe thrombocytopenia. Pre anaesthesia workup including a CBC and coagulation profile is a must. Increased intra and post operative bleeding can occur despite normal haematological parameters, so necessary blood products should be kept available. Substrate-reducing therapy may interfere with perioperative drug therapy. Eliglustat is Table 1: Summary of CBC and interventions done.Time Hb (g/dL) WBC count Platelet count (per cmm) Intervention6 weeks 9.4 4100 62,000 Nil13 weeks 8.4 3410 90,000 IV Iron FCM 1 g17 weeks 7.2 2690 1.2 lac IV Iron FCM 1 g24 weeks 8.6 2800 1.05 lac IV Iron FCM 1 gPre delivery 36 weeks 9.6 1.03 lac PCV reservedWBC: White blood cellFigure 1: Demarcating splenomegaly and hepatomegaly